Gabapentin Abuse
Showing: opioids

There are many variables regarding the analyses of substance abuse testing. Clients will often ask about specifics pertaining to the determination of time, dose and frequency when detecting substance(s) of abuse.
When testing a reservoir matrix- a material or substance which can accumulate and retain drug and alcohol biomarkers (eg., urine, blood, hair, nail, umbilical cord, or meconium, etc.), the reported quantitation of a drug or its metabolite cannot be used to determine when/if a specific substance was used, how much of a substance was used or how often a substance was used. Test results show only if a substance was detected or not detected.
A specimen’s window of detection provides an estimated timeframe for detecting substance(s) of abuse. Based on extensive research studies, the generally accepted windows of detection for specimens used in our testing are as follows:
- Scalp Hair- Up to approximately 3 months prior to collection.
- Fingernail- Up to approximately 3-6 months prior to collection.
- Umbilical Cord- Up to approximately 20 weeks prior to birth.
- Meconium- Up to approximately 20 weeks prior to birth.
- Urine- Up to approximately 2-3 days prior to collection.
- Blood (PEth)-May be up to approximately 2-4 weeks prior to collection.
It is important to know that the interpretation of drug testing results may be determined by a Medical Review Officer (MRO). A Medical Review Officer is a licensed physician (MD or DO) who has knowledge of substance abuse disorders and has the appropriate medical training to interpret and evaluate an individual’s positive test result together with his or her medical history and any other relevant biomedical information.1This is an incredibly important aspect of drug testing. A laboratory can detect substances, but an MRO may be used to interpret what that detection means.
1. Journal of Occupational and Environmental Medicine: (January 2003-Volume 45-Issue 1-p 102-103) Qualifications of Medical Review Officers (MRO’s) in Regulated and Nonregulated Drug Testing. Departments: ACOEM Consensus Opinion Statement
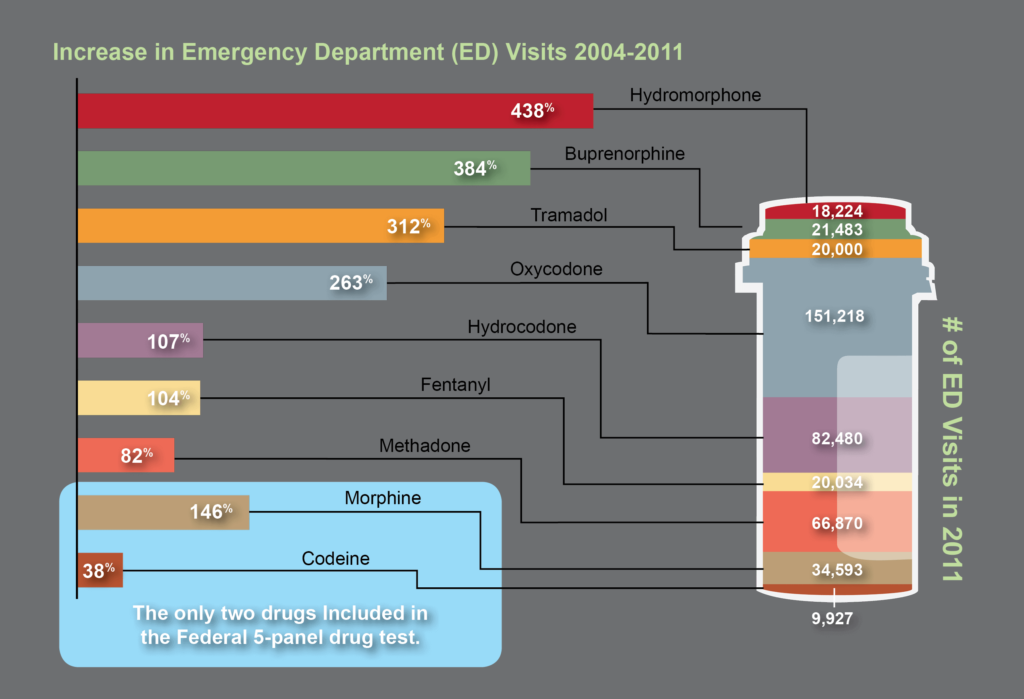
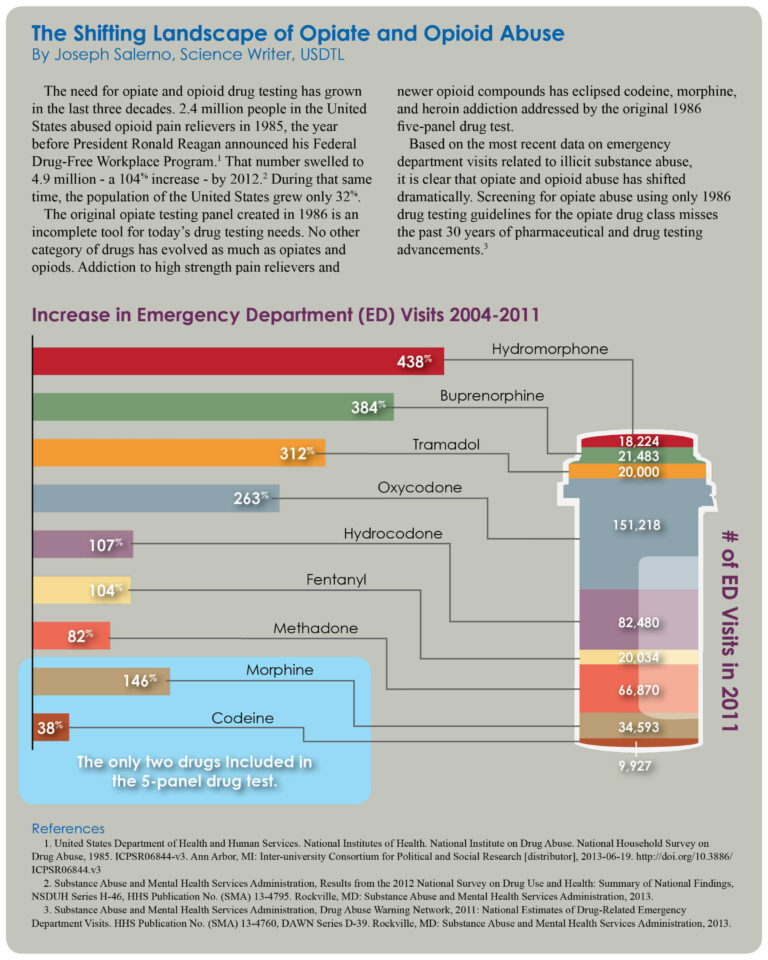
The need for opiate and drug testing has grown in the last three decades. 2.4 million people in the United States abused opioid pain relievers in 1985, the year before President Ronald Reagan announced his Federal Drug-Free Workplace Program.1 That number swelled to 4.9 million – a 104% increase – by 2012.2 During that same time, the population of the United States grew only 32%.
The original opiate testing panel created in 1986 is an incomplete tool for today’s drug testing needs. No other category of drugs has evolved as much as opiates and opioids. Addiction to high strength pain relievers and newer opioid compounds has eclipsed codeine, morphine, and heroin addiction addressed by the original 1986 five-panel drug test.
Based on the most recent data on emergency department visits related to illicit substance abuse, it is clear that opiate and opioid abuse has shifted dramatically. Screening for opiate abuse using only 1986 drug testing guidelines for the opiate drug class misses the past 30 years of pharmaceutical and drug testing advancements.3
References:
- Umbilical Cord Tissue Testing for SSRIs
- A Comparison of Turnaround-Times for Two Popular Specimen Types Used for Newborn Toxicology: Meconium and Umbilical Cord Tissue
- Using Umbilical Cord Tissue to Identify Prenatal Ethanol Exposure and Co-exposure to Other Commonly Misused Substances
- Toxicology as a Diagnostic Tool to Identify the Misuse of Drugs in the Perinatal Period
- Specimen Delay
- Drug Classes and Neurotransmitters: Amphetamine, Cocaine, and Hallucinogens
- Environmental Exposure Testing for Delta-8 THC, Delta-9 THC, Delta-10 THC, and CBD
- Bromazolam and Synthetic Benzodiazepines
- October 2024 (5)
- March 2024 (1)
- February 2024 (1)
- January 2024 (3)
- December 2023 (1)
- November 2023 (1)



