Limits of Interpreting A Drug Test
Showing: neontal drug exposure

There are many variables regarding the analyses of substance abuse testing. Clients will often ask about specifics pertaining to the determination of time, dose and frequency when detecting substance(s) of abuse.
When testing a reservoir matrix- a material or substance which can accumulate and retain drug and alcohol biomarkers (eg., urine, blood, hair, nail, umbilical cord, or meconium, etc.), the reported quantitation of a drug or its metabolite cannot be used to determine when/if a specific substance was used, how much of a substance was used or how often a substance was used. Test results show only if a substance was detected or not detected.
A specimen’s window of detection provides an estimated timeframe for detecting substance(s) of abuse. Based on extensive research studies, the generally accepted windows of detection for specimens used in our testing are as follows:
- Scalp Hair- Up to approximately 3 months prior to collection.
- Fingernail- Up to approximately 3-6 months prior to collection.
- Umbilical Cord- Up to approximately 20 weeks prior to birth.
- Meconium- Up to approximately 20 weeks prior to birth.
- Urine- Up to approximately 2-3 days prior to collection.
- Blood (PEth)-May be up to approximately 2-4 weeks prior to collection.
It is important to know that the interpretation of drug testing results may be determined by a Medical Review Officer (MRO). A Medical Review Officer is a licensed physician (MD or DO) who has knowledge of substance abuse disorders and has the appropriate medical training to interpret and evaluate an individual’s positive test result together with his or her medical history and any other relevant biomedical information.1This is an incredibly important aspect of drug testing. A laboratory can detect substances, but an MRO may be used to interpret what that detection means.
1. Journal of Occupational and Environmental Medicine: (January 2003-Volume 45-Issue 1-p 102-103) Qualifications of Medical Review Officers (MRO’s) in Regulated and Nonregulated Drug Testing. Departments: ACOEM Consensus Opinion Statement

By Adobe Stock©
According to the Centers for Disease Control and Prevention (CDC), fentanyl-related overdose deaths in the United States have increased by more than 1,000 percent from 2011 through 2016.1 The increased availability of illicitly manufactured fentanyl has contributed to the prevalence of neonatal abstinence syndrome (NAS) or neonatal opioid withdrawal syndrome (NOWS) throughout the United States. There was a greater than five-fold increase in the proportion of babies born with NAS from 2004 to 2014, when an estimated 32,000 infants were born with NAS/NOWS —equivalent to one baby suffering from opioid withdrawal born approximately every 15 minutes.2
In response to an ongoing opioid epidemic, medical professionals and advocates are invested in providing education, resources, testing, and preventative care in efforts to combat one of the most powerful opioids in the world —Fentanyl. This synthetic, opioid is extremely addictive and when used while pregnant can cause devastating outcomes to the fetus including neural tube defects, congenital heart defects, gastroschisis, stillbirth, and pre-term delivery.3
Newborns are often diagnosed with NAS/NOWS as a direct result of a sudden interruption of fetal exposure to prescription and illicit substances that were used or abused by the mother. Diagnosing an infant with NAS/NOWS is often assessed by identifying the following symptoms: tremors, jitteriness, irritability, excessive crying, and diarrhea. These indicators have been determined in the medical field as the standards for identifying a compromised central nervous system. Prenatal use & abuse of fentanyl can increase an infant’s risk for developing NAS/NOWS.
What Makes Fentanyl So Dangerous?
According to the United States Drug Enforcement Administration (DEA), fentanyl is approximately 100 times more potent than morphine and 50 times more potent than heroin.4 Its initial development was intended as a licit intravenous anesthetic; however, it has now become a public health threat as pharmaceutical products are subjected to theft, fraudulent prescriptions and illicit or less regulated distribution and manufacturing. The drug is often abused by injecting, snorting / sniffing, oral tablets/pills or removing the gel on fentanyl patches and then injecting or ingesting. The popularity of fentanyl within drug addicted communities derive from the addictive effects the drug produces —relaxation, euphoria, pain relief, and sedation. However, abused fentanyl also produces fatal side effects including: confusion, dizziness, nausea, vomiting, urinary retention, pupillary constriction, and respiratory depression.
Fentanyl’s Long-Term Effects on Fetus
Prenatal substance abuse can lead to long-term, irreversible damage to a developing fetus. In relation to fentanyl, these effects can consist of the following when associated with NAS/NOWS:5
- Cognitive and motor development deficiencies
- Infections
- Vision complications
- Sudden Infant Death Syndrome (SIDS)
- Susceptible to future substance abuse
Fentanyl and Toxicology Confirmation
According to the American Academy of Pediatrics, NAS/NOWS is a clinical diagnosis, however toxicological confirmation is necessary to identify the exact type of substance that the mother was using or abusing and to confirm or rule out the use of other licit or illicit substances during pregnancy.6
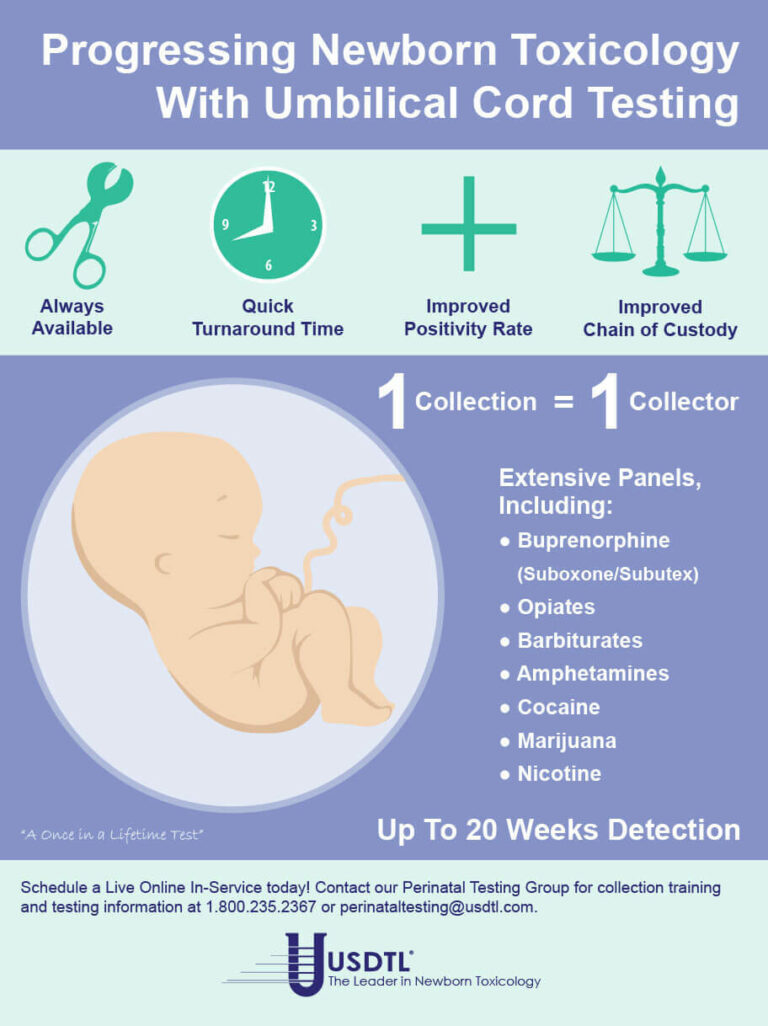
Due to the recent increase of cases regarding pregnant women and fentanyl exposure, it is critical to utilize testing that can help detect the latest drugs of abuse. Through internal research and strategic analysis, our laboratory is combating the growing epidemic of fentanyl use among pregnant women and those of childbearing age, with the development of testing for fentanyl in both umbilical cord tissue and meconium. As the first and only laboratory to offer fentanyl testing utilizing both advanced newborn specimens, our partnerships are provided with the opportunity to proactively and effectively address the alarming rates of prenatal fentanyl use. Both meconium and umbilical cord tissue belong to the baby, therefore maternal consent is not needed to proceed in testing.
Umbilical Cord Tissue
The umbilical cord tissue is available immediately for 100 percent of births and requires only 1 collection by 1 collector. This specimen’s look back provides detection up to approximately 20 weeks prior to birth, the most advanced newborn specimen on the market.
Meconium
Meconium has been recognized for decades as the “gold standard” for the detection of substances. It is the first stool of an infant produced in utero, consisting of epithelial cells, bile, mucous, and is odorless with a very dark, tar-like appearance. Meconium is uniquely developed during gestation with exclusive capabilities of preserving substances exposed prenatally up to approximately 20 weeks prior to birth. The average start of a meconium passage after birth occurs within 24-48 hrs., in most newborns it is generally passed in the first day or so of life.
Turnaround Time
Our methodology and advanced instrumentation contribute to our laboratory’s efficient turnaround time. Generally, the standard turnaround time for reporting negative screening test results is the next business day, with an additional 1-2 business days for specimens that require confirmatory testing. Turnaround time begins from receipt of the valid specimen–accompanied by a properly documented valid order– into the laboratory. Some tests require additional time to process and will fall outside the standard turnaround time window.
As one of the only laboratories in the nation focusing exclusively on substance abuse testing, we are consistently adapting our services to meet the needs of our clients and communities. The validity of newborn toxicology results has never been more imperative. Through our partnership and services, we can customize a flexible comprehensive drug testing program based on your population health needs. Today’s substance abuse landscape is drastically different than it used to be-collaboration is vital in supporting our mission of protecting and enriching lives.
References
- Spencer, M.P.H., M., Warner, Ph.D, M., Bastian, B.S., B., Trinidad, M.P.H., M.S., J. and Hedegaard, M.D., M.S.P.H., H. (2019). “National Vital Statistics Reports.” [online] Cdc.gov. Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_03-508.pdf [Accessed 17 Sep. 2019].
- Drugabuse.gov. (2019). “Dramatic Increases in Maternal Opioid Use and Neonatal Abstinence Syndrome.” [online] Available at: https://www.drugabuse.gov/related-topics/trends-statistics/infographics/dramatic-increases-in-maternal-opioid-use-neonatal-abstinence-syndrome [Accessed 17 Sep. 2019].
- Center for Disease Control and Prevention, U. (n.d.). “Pregnancy and Opioid Pain Medications.” Retrieved from https://www.cdc.gov/drugoverdose/pdf/pregnancy_opioid_pain_factsheet-a.pdf.
- Department of Education, U. (n.d.). “Drugs of Abuse Guide” (2017)(pp. 40-41) (United States).
- “Long-Term Outcomes of Infants With Neonatal Abstinence Syndrome.” (n.d.). Retrieved from https://www.seattlechildrens.org/healthcare-professionals/education/continuing-medical-nursing-education/neonatal-nursing-education-briefs/long-term-outcomes-of-infants-with-nas/
- Kocherlakota, P. (2014, August 01). “Neonatal Abstinence Syndrome.” Retrieved from http://pediatrics.aappublications.org/content/134/2/e547
What you need to know about meconium collection.
by Michelle Lach, MSIMC
Meconium is the first stool of a newborn infant. It is produced in utero and consists of materials such as epithelial cells, bile, mucous, and more. In most newborns, meconium is generally passed in the first day or so of life, has no odor, and appears as a very dark, tar-like substance. This helps distinguish meconium from the next phase of passage called transitional stool.
Transitional stool will start to have an odor and present with a more brown, green, or yellow color as the newborn starts digesting milk. When drug testing the meconium of a newborn, it is important to note this difference since only meconium is created during gestation and transitional stool is created after birth. Collection of any stool other than meconium for drug testing purposes may result in a rejected specimen.
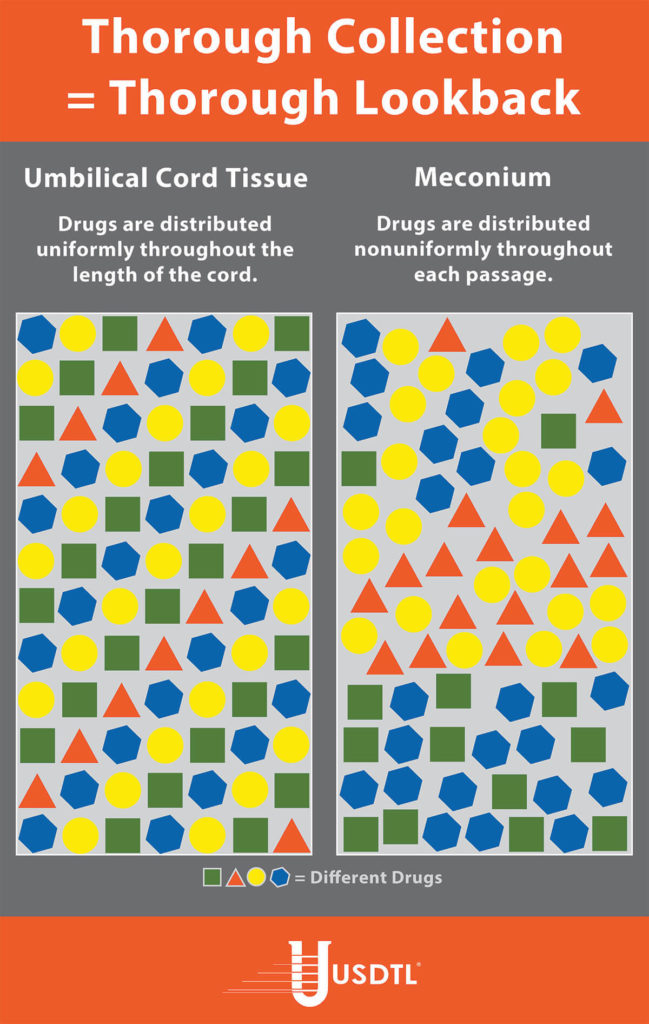
Unlike umbilical cord tissue, drugs are not distributed uniformly throughout the meconium specimen (see Figure 1). Because of this, the collection of the entire mass of meconium is highly encouraged to assure that there will be enough specimen to test, and that the maximum window of drug detection is achieved. It can take multiple passages of meconium before the newborn begins the transitional stool phase.
We require a minimum of 3 grams of meconium to be able to properly run our tests, so collecting the entire passage of meconium from newborns that have been exposed to substances of abuse is highly critical since they tend to have lower birth weights and create less specimen in the first place. If there is not enough specimen to run the test, the results are reported out as QNS. Quantity Not Sufficient (QNS) is a result of not having a sufficient quantity (volume) of specimen to test for the panels ordered.
Neonatal Drug Withdrawal
By Freepik© Studio
Maternal Nonnarcotic Drugs that cause neonatal psychomotor behavior consistent with withdrawal.
The Onset of Newborn Withdrawal Symptoms is Highly Variable
| Drug | Onset of Signs |
| Diazepam | Hours to Weeks |
| Alcohol | 3-12 Hours |
| Heroin | 24 Hours |
| Sedatives | 1-3 Days |
| Methadone | 1-7 Days |
| Opiates | 1-7 Days |
| Barbiturates | 1-14 Days |
– Click here to download the pdf.
Read an excerpt from the article Neonatal Drug Withdrawal below:
Signs characteristic of neonatal withdrawal have been attributed to intrauterine exposure to a variety of drugs. Other drugs cause signs in neonates because of acute toxicity. Chronic in utero exposure to a drug (eg, alcohol) can lead to permanent phenotypical and/or neurodevelopmental-behavioral abnormalities consistent with drug effect. Signs and symptoms of withdrawal worsen as drug levels decrease, whereas signs and symptoms of acute toxicity abate with drug elimination. Clinically important neonatal withdrawal most commonly results from intrauterine opioid exposure. The constellation of clinical findings associated with opioid withdrawal has been termed the neonatal abstinence syndrome (NAS). Among neonates exposed to opioids in utero, withdrawal signs will develop in 55% to 94%.1,2 Neonatal withdrawal signs have also been described in infants exposed antenatally to benzodiazepines,3,4 barbiturates,5,6 and alcohol.7,8
— Neonatal Drug Withdrawal https://doi.org/10.1542/peds.2011-3212
References:
-
Harper RG, Solish GI, Purow HM, Sang E, Panepinto WC. The effect of a methadone treatment program upon pregnant heroin addicts and their newborn infants. Pediatrics. 1974 ; 54 (3): 300–305 [PubMed]
-
Ostrea EM, Chavez CJ, Strauss ME. A study of factors that influence the severity of neonatal narcotic withdrawal. J Pediatr. 1976; 88 (4 pt 1): 642–645 [PubMed]
-
Rementería JL, Bhatt K. Withdrawal symptoms in neonates from intrauterine exposure to diazepam. J Pediatr. 1977; 90 (1): 123–126 [PubMed]
-
Athinarayanan P, Piero SH, Nigam SK, Glass L. Chloriazepoxide withdrawal in the neonate. Am J Obstet Gynecol. 1976; 124 (2): 212–213 [PubMed]
-
Bleyer WA, Marshall RE. Barbiturate withdrawal syndrome in a passively addicted infant. JAMA. 1972; 221 (2): 185–186 [PubMed]
- Desmond MM, Schwanecke RP, Wilson GS, Yasunaga S, Burgdorff I. Maternal barbiturate utilization and neonatal withdrawal symptomatology. J Pediatr. 1972; 80 (2): 190–197 [PubMed]
- Pierog S, Chandavasu O, Wexler I. Withdrawal symptoms in infants with the fetal alcohol syndrome. J Pediatr. 1977; 90 (4): 630–633 [PubMed]
- Nichols MM. Acute alcohol withdrawal syndrome in a newborn. Am J Dis Child. 1967; 113 (6): 714–715 [PubMed]

Numerous studies have shown that meconium specimens are too often unavailable for substance exposure testing. Universal collection of umbilical cord specimens offers a solution.
By Joseph Salerno
Unable, despite her best efforts to shake her addiction, a woman exposes her unborn child to drugs in the womb. The baby is born, healthy and beautiful with all the promise the future holds. Three days later, the withdrawal symptoms kick in. The baby wails, flush with the pains of withdrawal and inconsolable, unable to sleep, experiencing seizures. The NICU physician wants to know what the baby has been exposed to, but now it’s too late. The meconium has already been passed and discarded, and the umbilical cord is gone, lost opportunities for concrete answers. Now it’s a guessing game.
This isn’t just a “what-if” scenario, unfortunately, but a potential reality in a surprisingly large number of newborn substance exposure cases. Withdrawal symptoms in substance exposed newborns can be delayed up to three, five, even seven days after the baby is born. Cases of in utero barbiturate exposure may not manifest withdrawal signs until 14 days post-delivery. By that time it’s too late to test any of the baby’s specimens for biomarkers of substance exposure, because the specimens are gone.
Universal collection of umbilical cord specimens offers a solution to avoid this dilemma. Umbilical cord is the only universally available specimen for substance exposure testing. Numerous studies have shown meconium is not available for testing in up to 27% of births. Meconium may be passed in utero. In some cases, there is not enough meconium volume to test even when it is able to be collected.
And again, meconium may have been passed by the newborn and discarded well before they begin to exhibit withdrawal symptoms. Unfortunately, this can also be a problem when the signs of in utero substance exposure emerge after the umbilical cord has been discarded. Newborn urine testing is not a viable option in these cases, because urine provides only a 1-3 day window of detection for substance exposure biomarkers, compared to the 20 week look-back of umbilical cord.
Universal collection of umbilical cord specimens for every birth ensures there are no lost opportunities should the need for substance exposure testing arise. Umbilical cord collection is extremely easy, requiring very little additional effort during post delivery procedures. Only six inches of the cord is required for substance testing, taking up very little storage space.
Umbilical cord tissue is a very stable and reliable specimen. Cord tissue is stable up to 1 week at room temperature, and up to 3 weeks when refrigerated, without jeopardizing the testing results. This is ample time for the emergence of newborn withdrawal symptoms, even in the most extreme cases. Enough time to avoid a missed opportunity for real answers. Only one donor and one collector are present during the umbilical cord collection – in contrast to the multiple collections and multiple collectors involved with meconium – greatly improving chain-of-custody integrity. Umbilical cord specimens are ready for transport just minutes after the birth, greatly improving turnaround time for results reporting. Meconium passages can be delayed for days before being sent to the lab.
References
1. Arendt, R., Singer, L., Minnes, S. and Salvator, A. (1999). Accuracy in detecting prenatal drug exposure. Journal of Drug Issues. 29(2), 203-214.
2. Ostrea, E., Knapp, D., Tannenbaum, L., Ostrea, A., Romero, A., Salari, V. and Ager, J. (2001). Estimates of illicit drug use during pregnancy by maternal interview, hair analysis, and meconium analysis. Pediatrics. 138, 344-348.
3. Lester, B., ElSohly, M., Wright, L., Smeriglio, V., Verter, J., Bauer, C., Shankaran, S., Bada, H., Walls, C., Huestis, M., Finnegan, L. and Maza, P. (2001). The maternal lifestyle study: Drug use by meconium toxicology and maternal self-report. Pediatrics. 107(2), 309-317.
4. Derauf, C., Katz, A. and Easa, D.. (2003). Agreement between Maternal Self-reported Ethanol Intake and Tobacco Use During Pregnancy and Meconium Assays for Fatty Acid Ethyl Esters and Cotinine. American Journal of Epidemiology. 158, 705–709.
5. Eylera, F., Behnkea, M., Wobiea, K., Garvanb, C. and Tebb, I. (2005). Relative ability of biologic specimens and interviews to detect prenatal cocaine use. Neurotoxicology and Teratology. 27, 677 – 687.
Video links from “The Doctors” re: Newborns Exposed to Drugs And Alcohol in The Womb.
 Methamphetamine Babies
Methamphetamine Babies
Registered nurse Linda West and fellow Angels in Waiting nurses join The Doctorsto share their experiences fostering abandoned methamphetamine babies. OB/GYN Dr. Lisa Masterson explains how using methamphetamine while pregnant affects a growing fetus.
Angels In Waiting
More than 550,000 babies are born every year after exposure to drugs and alcohol in the womb. These babies are often born premature and with serious health issues, which make them “unpopular” for adoption. Registered nurse Linda West took matters into her own hands and founded the organization Angels in Waiting, a network of neonatal intensive care unit nurses who become foster parents for abandoned babies
How We Can Help
USDTL CordStat® definitively confirms opioid exposure. Many NAS babies are poly-substance exposed in utero. CordStat 12 and 13 drug panels identify the majority of opioids along with many drugs often associated with NAS. Positive results are an objective measure and often times the only flag that baby’s home life may need extra care. Go to www.USDTL.com to learn more.
 Q: What is Meconin and why is it important in newborn toxicology?
Q: What is Meconin and why is it important in newborn toxicology?
A: Morphine is the predominant metabolite of heroin, but morphine is also a stand alone drug and a metabolite of codeine. Some mothers are provided morphine during delivery. Historically, there have been instances where heroin using moms could not be distinguished from moms given morphine during delivery. Meconin is a contaminating constituent from poppy that is present in heroin. Therefore, like Monoacetylmorphine – a metabolite of heroin, the presence of Meconin indicates the use of heroin and when found in newborn specimens indicate fetal exposure to heroin.
USDTL offers Meconin testing in both umbilical cord tissue and meconium.
Click here to find out more about our testing services
Why USDTL requires Forensic Specimen Handling for all but research projects
 As you know, the primary specimen handling issues that differentiate a forensic from a clinical toxicology specimen are:
As you know, the primary specimen handling issues that differentiate a forensic from a clinical toxicology specimen are:
* securing the specimen with a tamper-evident seal
* a documented chain of custody.
A positive test result may do more than affect the newborn’s treatment. Today, a further consequence of a positive newborn toxicology report may involve intervention by the State due to a significant number of jurisdictions requiring reporting of all positive newborn toxicology results. In some instances, these positive results may ultimately lead to termination of parental rights. Therefore, it is very important that all newborn specimens be handled as if they may be litigated. It is this distinct possibility of litigation that is the driving force behind USDTL’s requirement for proper chain-of-custody handling. It is also the reason why CAP-accredited laboratories follow forensic procedures in all areas including newborn toxicology services.
The minimum criteria to ensure the integrity of the chain-of-custody for a specimen is the presence of an intact tamper-evident seal and the signature and date of the collector or the individual that prepared the specimen for send out. A tamper-evident seal is supplied with each USDTL Chain-of-Custoday and Control Form for your convenience. Our concern is in the best interests of our smallest patients.
- Hair, Nail, and Umbilical Cord Testing for Phenibut, Medetomidine, and Tianeptine
- Umbilical Cord Tissue Testing for SSRIs
- A Comparison of Turnaround-Times for Two Popular Specimen Types Used for Newborn Toxicology: Meconium and Umbilical Cord Tissue
- Using Umbilical Cord Tissue to Identify Prenatal Ethanol Exposure and Co-exposure to Other Commonly Misused Substances
- Toxicology as a Diagnostic Tool to Identify the Misuse of Drugs in the Perinatal Period
- Specimen Delay
- Drug Classes and Neurotransmitters: Amphetamine, Cocaine, and Hallucinogens
- Environmental Exposure Testing for Delta-8 THC, Delta-9 THC, Delta-10 THC, and CBD
- February 2025 (1)
- October 2024 (5)
- March 2024 (1)
- February 2024 (1)
- January 2024 (3)
- December 2023 (1)


