Limits of Interpreting A Drug Test
Showing: drugs in fingernail

There are many variables regarding the analyses of substance abuse testing. Clients will often ask about specifics pertaining to the determination of time, dose and frequency when detecting substance(s) of abuse.
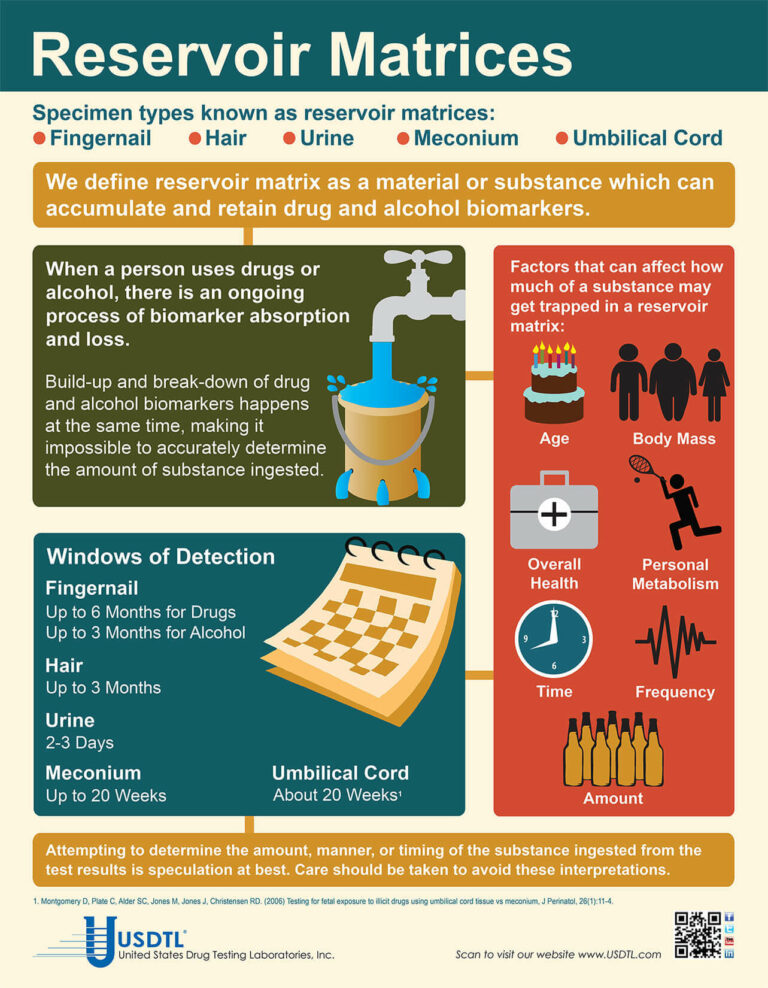
When testing a reservoir matrix- a material or substance which can accumulate and retain drug and alcohol biomarkers (eg., urine, blood, hair, nail, umbilical cord, or meconium, etc.), the reported quantitation of a drug or its metabolite cannot be used to determine when/if a specific substance was used, how much of a substance was used or how often a substance was used. Test results show only if a substance was detected or not detected.
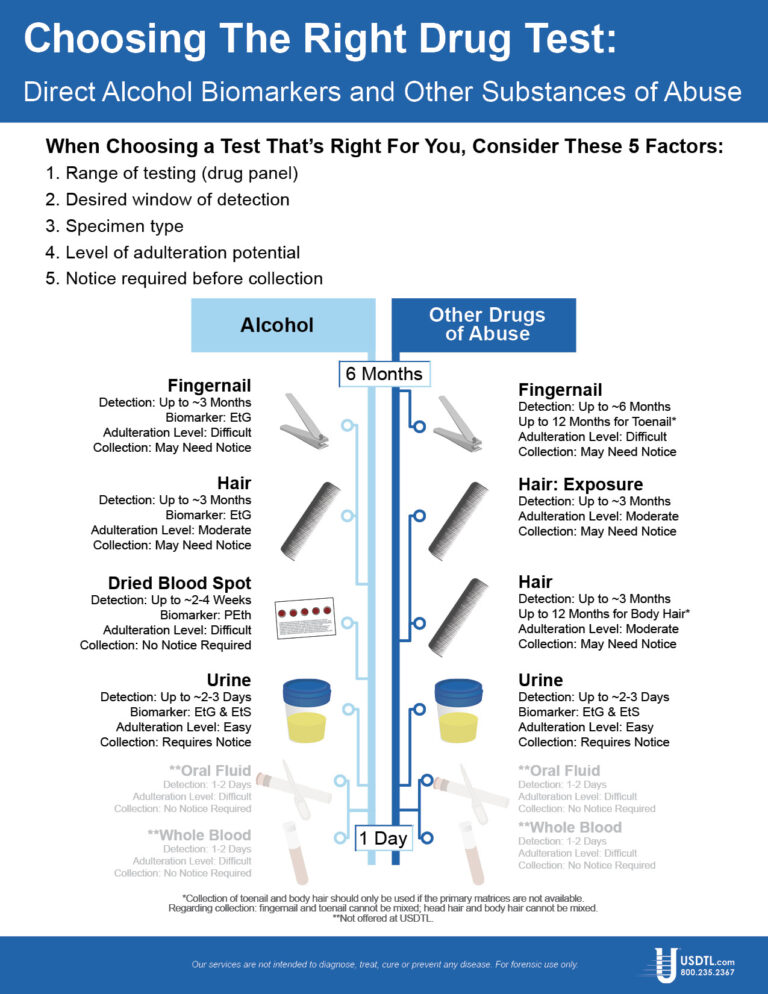
A specimen’s window of detection provides an estimated timeframe for detecting substance(s) of abuse. Based on extensive research studies, the generally accepted windows of detection for specimens used in our testing are as follows:
- Scalp Hair- Up to approximately 3 months prior to collection.
- Fingernail- Up to approximately 3-6 months prior to collection.
- Umbilical Cord- Up to approximately 20 weeks prior to birth.
- Meconium- Up to approximately 20 weeks prior to birth.
- Urine- Up to approximately 2-3 days prior to collection.
- Blood (PEth)-May be up to approximately 2-4 weeks prior to collection.
It is important to know that the interpretation of drug testing results may be determined by a Medical Review Officer (MRO). A Medical Review Officer is a licensed physician (MD or DO) who has knowledge of substance abuse disorders and has the appropriate medical training to interpret and evaluate an individual’s positive test result together with his or her medical history and any other relevant biomedical information.1This is an incredibly important aspect of drug testing. A laboratory can detect substances, but an MRO may be used to interpret what that detection means.
1. Journal of Occupational and Environmental Medicine: (January 2003-Volume 45-Issue 1-p 102-103) Qualifications of Medical Review Officers (MRO’s) in Regulated and Nonregulated Drug Testing. Departments: ACOEM Consensus Opinion Statement
Dear Valued Client,
We are proud to announce that we are the first laboratory in the world to be ISO/IEC 17025 accredited for drug and alcohol testing in umbilical cord, fingernail, and toenail specimens. On September 4, 2015, USDTL attained ISO/IEC 17025 accreditation showing full compliance with the international testing standards. We have received our accreditation from ANSI-ASQ National Accreditation Board, demonstrating technical competence in the field of forensic testing. The scope of our ISO/IEC 17025 accreditation encompasses all specimen types and methods of analysis utilized in our laboratory.
Our laboratory has always maintained this level of quality and competency since our humble beginnings in 1991, bringing our clients the most responsive, personal service in the drug and alcohol testing industry. ISO/IEC 17025 accreditation reaffirms that commitment to our clients, for all aspects of our testing and client advocacy. You can always have absolute confidence that the results of every specimen tested by our laboratory will meet the highest of international standards.
ISO/IEC 17025 is the single most important standard applied to testing and calibration laboratories around the globe. Laboratories accredited to the ISO/IEC 17025 standard have demonstrated that they are technically competent and able to reproducibly generate accurate, precise and consistent data.
The practical benefits for clients of USDTL of ISO/IEC 17025 accreditation are seen on a continual basis:
- Continuously produced testing results of the highest quality, validity, and integrity;
- Improved customer communication and resolution of customer issues;
- Continual improvement of our management system, with an emphasis on the responsibilities of senior management;
- Fast resolution of laboratory issues regarding methods and equipment.
- Evidential acceptance of USDTL laboratory results in virtually all jurisdictions.
To view our certificate of accreditation by ANAB 17025:2017 Forensic Science Testing and Calibration Lab. If you have questions about ISO/IEC 17025 accreditation, please contact us at clientservices@usdtl.com or 800.235.2367.
Sincerely,
Adam Negrusz, Ph.D., F-ABFT
Laboratory Director

 Des Plaines, IL – United States Drug Testing Laboratory, Inc. (USDTL), a forensic laboratory specializing in drug and alcohol testing using advanced specimens, has released a new assay to detect zolpidem (Ambien®) use in fingernail and hair specimens. Previously available only in urine and oral-fluid specimens, zolpidem testing in fingernails and hair offers forensic drug testing professionals new, powerful tools to meet their drug testing needs.
Des Plaines, IL – United States Drug Testing Laboratory, Inc. (USDTL), a forensic laboratory specializing in drug and alcohol testing using advanced specimens, has released a new assay to detect zolpidem (Ambien®) use in fingernail and hair specimens. Previously available only in urine and oral-fluid specimens, zolpidem testing in fingernails and hair offers forensic drug testing professionals new, powerful tools to meet their drug testing needs.
Since its approval in 1993 for the treatment of insomnia, zolpidem has become one of the most popular and most prescribed sleep aids. Zolpidem is a sedative-hypnotic medication that affects the same areas of the brain as benzodiazepines. In 2011, 39 million prescriptions for zolpidem products were written in the United States. [1]
Zolpidem use carries some risks of harm, especially when taken in combination with other substances. The number of emergency department visits related to zolpidem grew by 136% from 2004 (12,792) to 2011 (30,149). [2] Other substances were involved in 57% of those ED visits, including benzodiazepines (26%), narcotic pain relievers (25%), and alcohol (14%). [3]
The development of zolpidem testing is driven by USDTL’s ongoing commitment to be a leader in substance abuse and alcohol detection. Zolpidem testing in fingernails and hair will be available on October 1st, 2014.
USDTL has made significant breakthroughs in detecting alcohol and other substances of abuse. They offer a wide range of testing services and specialize in hard to detect substances of abuse, customized assays, and advanced drug testing specimens.
References
1. IMS, Vector One: National (VONA) and Total Patient Tracker (TOT). Year 2011. Extracted June 2011.
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. (May 1, 2013). Emergency Department Visits for Adverse Reactions Involving the Insomnia Medication Zolpidem. Rockville, MD.
3. Substance Abuse and Mental Health Services Administration, Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
- Hair, Nail, and Umbilical Cord Testing for Phenibut, Medetomidine, and Tianeptine
- Umbilical Cord Tissue Testing for SSRIs
- A Comparison of Turnaround-Times for Two Popular Specimen Types Used for Newborn Toxicology: Meconium and Umbilical Cord Tissue
- Using Umbilical Cord Tissue to Identify Prenatal Ethanol Exposure and Co-exposure to Other Commonly Misused Substances
- Toxicology as a Diagnostic Tool to Identify the Misuse of Drugs in the Perinatal Period
- Specimen Delay
- Drug Classes and Neurotransmitters: Amphetamine, Cocaine, and Hallucinogens
- Environmental Exposure Testing for Delta-8 THC, Delta-9 THC, Delta-10 THC, and CBD
- February 2025 (1)
- October 2024 (5)
- March 2024 (1)
- February 2024 (1)
- January 2024 (3)
- December 2023 (1)