Blog

By Freepik© Studio
With a recent rise in Bromazolam in the news, it’s important to look at what synthetic benzodiazepines are and how they can be dangerous.
What is Bromazolam?
Triazolobenzodiazepine is a synthetic benzodiazepine drug also known as bromazolam. It was originally developed in 1976, but it was never sent to market. It is considered a Novel Psychoactive Substance (NPS), meaning it does have some psychoactive effects along with the sedative effects of other benzodiazepines1.
Bromazolam is sold as tablets or powders, which means it can be used to adulterate drugs such as fentanyl or Alprazolam (Xanax®). Novel psychoactive substances belonging to the benzodiazepines class are usually purchased on the online drug market under various street names, such as “legal benzodiazepines”, “designer benzodiazepines” and “research chemicals”1. The trends from the European Monitoring Centre for Drugs and Drug Addiction showed warning signs for Bromazolam being sold illicitly on the streets in 20162.
Learn more about designer benzodiazepines in our blog post by USDTL’s Research and Development Coordinator, Amy Racines.

By Freepik© Studio
Benzodiazepines and Pregnancy
The FDA has categories for drugs and related pregnancy risks. Benzodiazepines are considered a category D, which means that positive evidence of risk exists, but there are benefits from use that may outweigh the risk1. These drugs include Alprazolam, Chlordiazepoxide, Clonazepam, Diazepam, and Lorazepam. Drugs that are contraindicated in pregnancy, or should not be given to pregnant women, are estazolam, fluazepam, quazepam, temazepam and triazolam1.
Neonatal withdrawal reactions have been observed in newborns after maternal benzodiazepine use in the last weeks of pregnancy. There is also concern that neonatal exposure during breastfeeding may lead to central nervous system depression1. Since Bromazolam is a benzodiazepine, it is important to keep these FDA categories in mind as there is more exposure to it.
Bromazolam in the News
There have been more articles highlighting the incoming danger of bromazolam being sold illicitly in the United States, sometimes falsely marketed as Xanax or Alprazolam. As of this blog post, here are three relevant news articles:
- August 29, 2023 – Indiana health officials warn about ‘emerging threat’ of drug Bromazolam
- August 29, 2023 – ‘Fake Xanax:’ A worry to law enforcement, often mixed with deadly fentanyl
- June 10, 2023 – Deadly drug just ‘a click away’, warns grieving Ontario family
References
- Critical Review Report: Bromazolam, World Health Organization
- European Drug Report 2023: Trends and Developments

Updated: 1/22/24:
We are running behind, but working overtime to get you results.
-
Due to weather conditions on Friday (1/12/24), we experienced a power outage at the laboratory. Our instrumentation, network (email, phones, etc.), and web portal were down late into the evening. Unfortunately, this means we experienced delays in receiving and processing specimens into the laboratory, causing delays in reporting some results.
-
FedEx is experiencing weather and other service delays, causing issues with getting specimens to our laboratory. You can learn more about FedEx delays here.
-
This morning, a pipe burst in the lab, adding more delay to our ability to process specimens.
This unfortunate collection of events has been challenging, but rest assured we are doing our best to process specimens as quickly as we can and get your reports to you as soon as possible.
Thank you for understanding. If you are also affected by the weather conditions, please be safe out there
Tianeptine is an atypical tricyclic antidepressant, commonly referred to as Gas Station Heroin or Za Za. Its use, linked to serious harm and death, has been making headlines. Tianeptine is not approved in the United States but is legal in other countries to treat depression or anxiety. Reports in the United States about severe harm due to tianeptine have been increasing, leading the FDA to issue a formal warning about the drug1.
 The clinical effects of tianeptine are very similar to opioids, leading some opioid users to switch to tianeptine as an opioid alternative1. Other users have turned to tianeptine to self-treat depression or anxiety. Tianeptine users experience dependence, withdrawal, and overdose when used at concentrations above the therapeutic doses prescribed in other countries1. However, when tianeptine is used in small doses consistent with therapeutic treatments, no negative side effects have been reported. Several case studies have shown that tianeptine toxicity mimics that experienced from opioids and that naloxone is an effective therapy2,3.
The clinical effects of tianeptine are very similar to opioids, leading some opioid users to switch to tianeptine as an opioid alternative1. Other users have turned to tianeptine to self-treat depression or anxiety. Tianeptine users experience dependence, withdrawal, and overdose when used at concentrations above the therapeutic doses prescribed in other countries1. However, when tianeptine is used in small doses consistent with therapeutic treatments, no negative side effects have been reported. Several case studies have shown that tianeptine toxicity mimics that experienced from opioids and that naloxone is an effective therapy2,3.
The Centers for Disease Control reports that tianeptine use is on the rise. From 2000 to 2014, there were 11 calls to poison control regarding tianeptine, but from 2014-2017, there were over 200 calls4. Nationally, tianeptine is not scheduled, though Alabama and Michigan have added the drug as a Schedule II substance. Tianeptine is readily available online, typically in tablet or powder form5.
References:
- Tianeptine Products Linked to Serious Harm, Overdoses, Death | FDA
- Acute Toxicity From Intravenous Use of the Tricyclic Antidepressant Tianeptine – PubMed (nih.gov)
- Amitriptyline and tianeptine poisoning treated by naloxone – PubMed (nih.gov)
- Characteristics of Tianeptine Exposures Reported to the National Poison Data System — United States, 2000–2017 | MMWR (cdc.gov)
- Tianeptine (usdoj.gov)

By Freepik© Studio
Kenosha County, Wisconsin, was one of the first counties in Wisconsin – and Wisconsin is still the only state in the nation – to use direct biomarker testing to help the population of impaired drivers served by the local assessment agency, the Hope Council on Alcohol and Other Drug Abuse, where I was Executive Director. Starting in July 2011 with the help of Pamela Bean, PhD, who had dedicated herself to reducing the number of drunk drivers on Wisconsin’s roads, the Hope Council, partnering with USDTL, implemented testing of convicted impaired drivers who were assessed for three or more offenses. In 2014 we expanded testing to include any person convicted and assessed for any number of offenses who was referred to treatment.
Testing the intoxicated driver population was the single most impactful change made in this programming since assessments were mandated in the late 1970s, and I truly don’t understand how every county in every state in the nation hasn’t jumped on board.
Dr. Bean laid the groundwork for Wisconsin counties by securing permission for the use of direct biomarker testing, but these aren’t just any tests. These tests specifically identify those who use alcohol problematically. We aren’t looking to “catch” drinkers. We’re looking to help those who have significant substance use disorders to recognize that their use is problematic and then guide them into treatment and provide tools for them to begin a life of abstinence…or at least to not drink during the Driver Safety Plan (DSP) that’s developed with them at the assessment.

Clients at the Hope Council with three or more offenses are required to have at least three Ethyl Glucuronide (EtG) tests during the one-year duration of their DSPs. EtG is detected in fingernails and shows a detection window of up to approximately 3-months of use. For those clients who have deliberately underplayed their use at the time of assessment, it’s likely that a follow-up Phosphatidylethanol (PEth) test would be scheduled within a month of the surprise positive test results. The PEth test uses dried blood spots and shows a detection window up to approximately 2-4 weeks of use. Many clients are surprised by the quality of these tests, so they may not be truthful at the assessment. The follow-up PEth allows the clients the opportunity to show that they are abstaining, as required by the assessment.
And if they aren’t? Well, that gives the assessment agency the opportunity to provide additional support and oversight for those who are having a hard time remaining abstinent. Addiction is a chronic, progressive, lethal disease, and it needs to be treated as such. Every other disease uses tests to provide valuable information for disease management, so why not the disease of addiction? Finger sticks for diabetes determine if blood sugar levels are balanced. Blood tests for international normalized ratio (INR) for heart disease determine if the blood is clotting properly. Direct biomarker tests should be used to determine if those assessed as having significant substance use disorders are maintaining appropriate levels of substances – specifically none – in their systems.
These tests are the best tools to help those suffering from the disease of addiction to recognize it and move toward treating it.
Click here to learn more on Guided by Guida. Click here to learn more about Alcohol by the Numbers.
Sign up here to learn more and receive our newsletter on the latest updates with USDTL.

By Freepik© Studio
1) What is the difference between a clinical test and a forensic test?
- Forensic testing includes confirmation testing, which uses a second portion/aliquot of the original specimen run on different, more sophisticated instruments when possible to duplicate positive screening results. This is a fail-safe to prevent false positives.
- Forensic testing also includes a clearly documented chain of custody, which ensures the specimen collected is the specimen that was tested and, in turn, reported out. This is because forensic testing has processes to ensure that the evidence created is defensible in a court of law, potentially mitigating issues associated with the testing.
2) How do I know if a laboratory is a good option for forensic newborn drug testing?
You should seek a laboratory that has forensic accreditations. Forensic drug testing laboratories work with the oversight of forensic accrediting bodies. These accrediting bodies review the laboratory processes regularly for accuracy and compliance. Laboratories that don’t have proper oversight can put an organization’s reputation and integrity at risk if their processes are determined to be forensically indefensible. This can lead to extensive and costly reevaluation. USDTL holds one of the highest international accreditations for forensic toxicology as an ANSI-ASQ National Accreditation Board (ANAB) ISO 17025 forensic testing laboratory. This means that every test completed by USDTL is done under the highest standards available for a forensic drug testing laboratory.
3) What is the best way to reduce the chances of missing newborn drug exposure while reducing testing bias?
Standardized collection of umbilical cord tissue at every birth ensures equity in the newborn drug testing process. No newborn will be left without the documented exposure they need for their lifetime and no hospital is left without the data they need to make the best decisions. This process streamlines staff training and reduces issues of missed collection because every umbilical cord is collected even if it is not tested. Stored specimens can be sent out for testing based on hospital protocol or if the neonate starts showing unexpected symptoms of withdrawal hours or days after birth. Specimens that do not need testing are simply discarded. See our blog The Onset of Newborn Withdrawal Symptoms is Highly Variable, which shows the different times onset can occur. According to the study, some barbituates could take up to 14 days for withdrawal to present.
By Canva© Studio
The Center for Disease Control and Prevention defines substance use disorders (SUDs) as ‘treatable, chronic diseases characterized by a problematic pattern of use of a substance or substances leading to impairments in health, social function, and control over substance use’1. Addiction at times can be incorrectly thought of as ‘a moral failing instead of what we know it to be: a chronic treatable brain disease’2, which results in the body sending signals in the absence of the drug. These signals cause the user to ‘crave’ the substance, much like the body sends signals when we are hungry or tired. These physiological changes are related to our body’s natural messengers, neurotransmitters.

Neurotransmitters are the body’s natural chemical messengers that control bodily functions such as breathing, heartbeat and blood pressure, muscle movement, sleep, digestion, and thoughts and memories3. Some examples of neurotransmitters are dopamine, glutamate, serotonin, norepinephrine, GABA, epinephrine, and histamine. The volume and storage of neurotransmitters within the body is highly regulated. The body can sense when there is too little or too much of a neurotransmitter and responds by creating more or destroying the neurotransmitter. Any change of this homeostasis can have severe consequences on the body’s ability to react to external stimuli and can be referred to as intoxication4.
The effects experienced by drugs is related to neurotransmitters. Neurotransmitters work on the body by binding to receptors. Neurotransmitters and their receptors work very similarly to a lock and key. The neurotransmitter perfectly fits into a specific receptor just like a key fits into a specific lock.

Some drugs, referred to as agonists, mimic neurotransmitters causing the body to think neurotransmitters are present, when in fact they are not. In our lock and key model, this would be like using an object to pick a lock in the absence of a key.
Some other drugs can increase or decrease the level of natural neurotransmitters in the body resulting in an increased or decreased level of signals. For example, the drugs can ‘copy’ the set of keys, allowing the lock to be opened more freely, or destroy the keys, preventing the lock from being opened.

Other drugs, called antagonists, block the body’s natural neurotransmitters causing no signal even when the stimuli are present. In our model, this would be equivalent to breaking a key off in the lock, preventing future keys from opening the lock (infographic 4). The effects of the drugs can be desirable such as euphoria or relaxation, while others are not desirable, like paranoia or increased blood pressure.

Over time with repeated drug usage, the body will compensate for the imbalance of neurotransmitters to restore the homeostasis. For instance, amphetamine increases the level of dopamine in the body. With chronic amphetamine use, the body recognizes the unnaturally high levels of dopamine. As a response, the body starts producing less dopamine. If the user then suddenly stops using amphetamine, they can experience side effects of low dopamine in the body, such as lack of motivation, lethargy, moodiness, or difficulty sleeping5. This phenomenon is referred to as withdrawal, and at times, for some substances, can be life-threatening.

Tolerance is similar to withdrawal in that it is also related to the body compensating for the imbalance of neurotransmitters. In the prior example, chronic amphetamine use causes the body to produce less dopamine. However, the ‘high’ experienced by amphetamine use is caused by the unnaturally high level of dopamine. If the body is producing less dopamine naturally, the user will need to take more amphetamine to experience the same ‘high’. This phenomenon is referred to as tolerance. Both tolerance and withdrawal are related to imbalance of the body’s neurotransmitters.
SUDs are more than just the psychological need to use a substance. They are complex diseases which affect the natural chemistry of the user’s body. Due to this complexity, individuals suffering from SUDs are recommended to seek treatment from professionals.
References
- https://www.cdc.gov/dotw/substance-use-disorders/index.html#:~:text=Substance%20Use%20Disorders%20(SUDs)%20are,and%20control%20over%20substance%20use.
- https://nida.nih.gov/nidamed-medical-health-professionals/health-professions-education/words-matter-language-showing-compassion-care-women-infants-families-communities-impacted-substance-use-disorder
- https://my.clevelandclinic.org/health/articles/22513-neurotransmitters#:~:text=Neurotransmitters%20are%20chemical%20messengers%20that,muscle%20cell%20or%20a%20gland.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3628948/#:~:text=Neurotransmitter%20expression%20can%20be%20transient,compensation%20to%20the%20changing%20environment.
- https://my.clevelandclinic.org/health/articles/22588-dopamine-deficiency
Sign up here to learn more and receive our newsletter on the latest updates with USDTL.
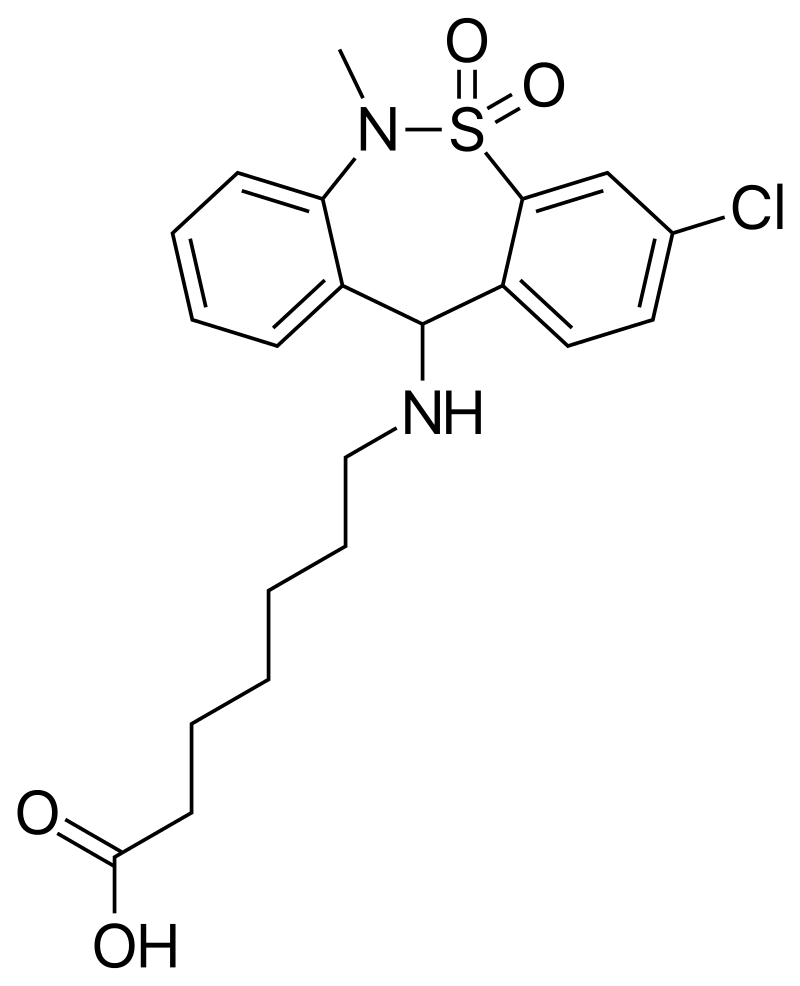
Benzodiazepines are a group of drugs which act as a central nervous system depressant and are used for the treatment of insomnia and anxiety. Although benzodiazepines are prescribed by doctors, they are also misused, most commonly in combination with opioids and alcohol. USDTL is excited to announce the addition of several benzodiazepines to the benzodiazepine panel in our umbilical cord assay beginning July 5th, 2023. Two of these added analytes are the designer benzodiazepines etizolam and flualprazolam, which can be purchased online.
The structures of medically approved benzodiazepines can be modified to create new compounds, commonly referred to as designer benzodiazepines, which are not medically approved. Etizolam and flualprazolam are two such designer benzodiazepines. Many times, these designer benzodiazepines are more potent than the medical benzodiazepines. Additionally, changing the structure to create these designer benzodiazepines affects the ability of this drug to be detected. Therefore, laboratories must update their methods to effectively detect new designer benzodiazepines.
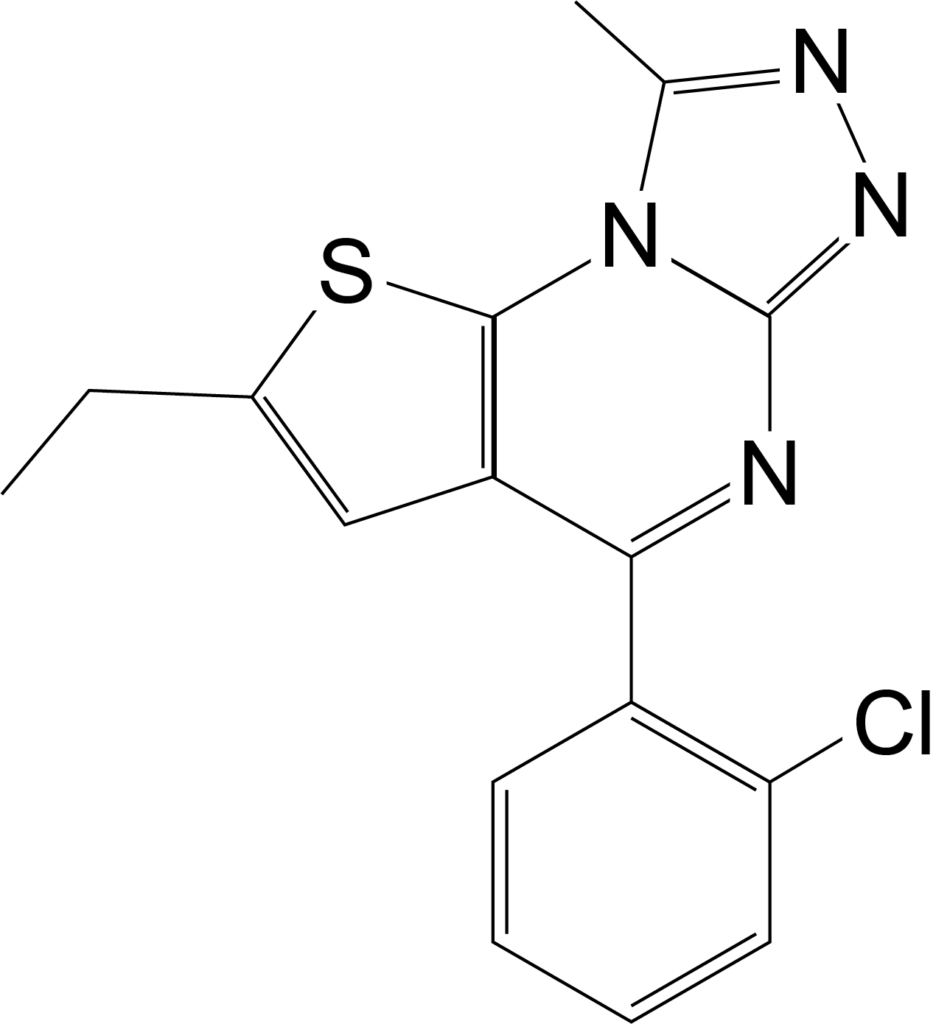 Etizolam has recently emerged in the illegal drug market in Europe and the United States, but it is an approved medication in Japan, India and Italy1. In 2018, the DEA reported 1,716 cases involving etizolam, but in 2021 that number rose to 4,252. Clinical studies have indicated that etizolam is about 10 times more potent than the medical benzodiazepine, diazepam. Common negative side effects of etizolam include drowsiness, sedation, muscle weakness and incoordination, fainting, headache, confusion, depression, slurred speech, visual disturbances, and changes in libido1.
Etizolam has recently emerged in the illegal drug market in Europe and the United States, but it is an approved medication in Japan, India and Italy1. In 2018, the DEA reported 1,716 cases involving etizolam, but in 2021 that number rose to 4,252. Clinical studies have indicated that etizolam is about 10 times more potent than the medical benzodiazepine, diazepam. Common negative side effects of etizolam include drowsiness, sedation, muscle weakness and incoordination, fainting, headache, confusion, depression, slurred speech, visual disturbances, and changes in libido1.
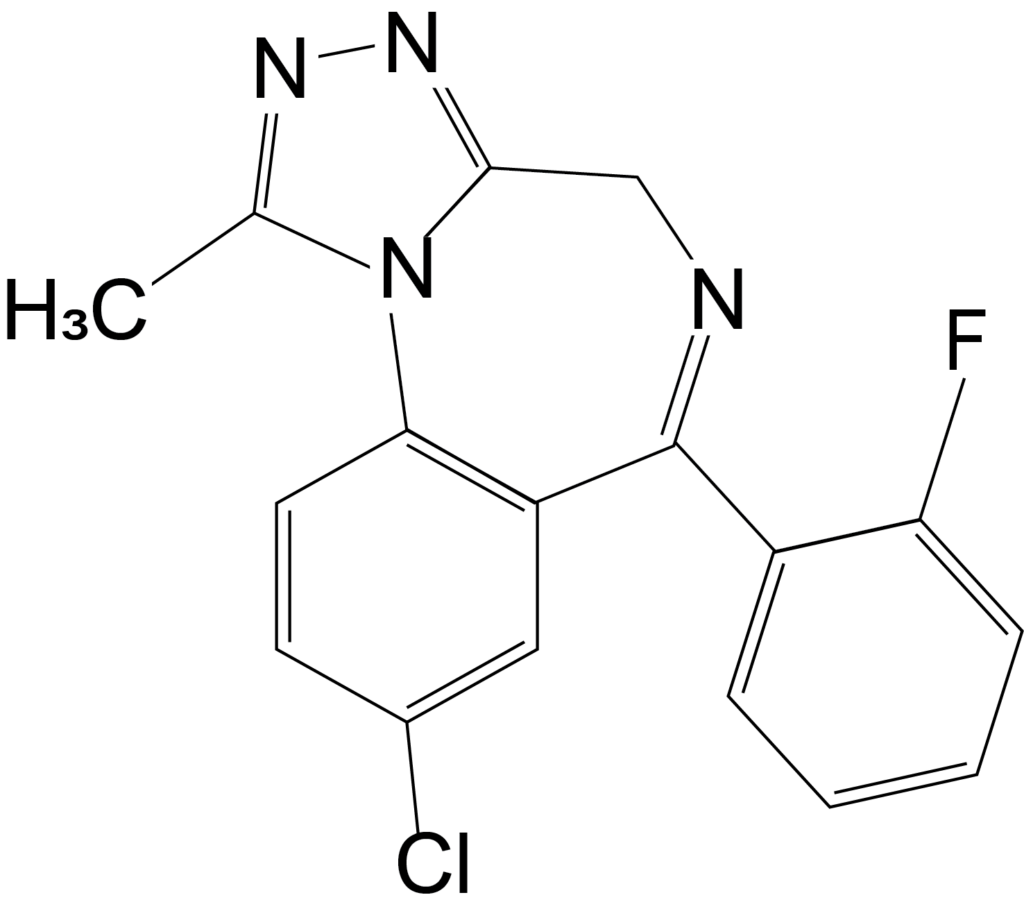 Flualprazolam is another such designer benzodiazepine which is not approved for use in the United States. Less is known about the potency of flualprazolam, but it is thought to create severe sedation and coma2. Flualprazolam is thought to be misused for its sedative and hypnotic effects, and users report the effects are similar to clonazepam and alprazolam2. The DEA only reported one case involving flualprazolam in 2017, but there were 1,624 cases in 2019.
Flualprazolam is another such designer benzodiazepine which is not approved for use in the United States. Less is known about the potency of flualprazolam, but it is thought to create severe sedation and coma2. Flualprazolam is thought to be misused for its sedative and hypnotic effects, and users report the effects are similar to clonazepam and alprazolam2. The DEA only reported one case involving flualprazolam in 2017, but there were 1,624 cases in 2019.
The DEA released an intent to temporarily schedule these two designer benzos as Schedule 1 substances in December 20223.
References:
- ETIZOLAM (usdoj.gov)
- Flualprazolam (Street Name: Flualp) (usdoj.gov)
- Federal Register :: Schedules of Controlled Substances: Temporary Placement of Etizolam, Flualprazolam, Clonazolam, Flubromazolam, and Diclazepam in Schedule I
By Canva© Studio
Diphenhydramine is an antihistamine often used to treat allergies, insomnia, and other conditions. However, there has been an alarming rise in diphenhydramine misuse in recent years, particularly among young people. The “Benadryl Challenge” on TikTok led the FDA to put out a statement in 2020 warning about the risk of using high doses.
What is Diphenhydramine?
Diphenhydramine (also known as DPH, Dimedrol, and Benadryl) is an antihistamine commonly used to treat allergy symptoms such as itching, sneezing, and runny nose. It is also used as a sleep aid and to treat other conditions such as motion sickness and Parkinson’s disease.
What are the Risks of Diphenhydramine Misuse?
Diphenhydramine is often misused in two ways, to induce sleep and to experience euphoria. Misusing diphenhydramine can have serious consequences. When taken in large doses, it can cause hallucinations, delirium, and seizures. It can also cause rapid heartbeat, high blood pressure, and difficulty breathing. In some cases, it can even be fatal.
In addition to the physical risks, diphenhydramine misuse can also have psychological effects. People who misuse the drug may experience anxiety, paranoia, and other mental health issues.
Are There Drug Tests for Diphenhydramine?
Yes. Though diphenhydramine is a legal substance, like alcohol, misuse can become a larger problem for some individuals, especially if they are in legal programs to get on the road to recovery. In these circumstances, drug testing for any mind-altering drug might be a consideration regardless of its status as legal or illicit.
USDTL offers diphenhydramine testing in hair and nail specimens to organizations that administer drug testing. Diphenhydramine can build up and remain in hair and nail specimens for up to approximately three months.
If your organization would benefit from offering this type of detection, please get in touch with us at 1-800-235-2367 or fill out our contact us form. Please note we are a business-to-business forensic laboratory only, and we do not offer toxicology testing to the general public. Due to confidentiality obligations, we cannot answer questions from parties not explicitly authorized by our business-to-business clients.
References:
1. U.S. Food and Drug Administration. (2019). Diphenhydramine (Benadryl): Drug Safety Communication – Serious Problems with High Doses from Over-the-Counter (OTC) Use. Retrieved from https://www.fda.gov/safety/medwatch-safety-alerts-human-medical-products/diphenhydramine-benadryl-drug-safety-communication-serious-problems-high-doses-over-counter-otc-use
2. National Institute on Drug Abuse. (2021). Misuse of Prescription Drugs. Retrieved from https://www.drugabuse.gov/publications/drugfacts/prescription-cns-depressants
3. Substance Abuse and Mental Health Services Administration. (2020). Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Retrieved from https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR1PDFW090120.pdf
4. American Academy of Pediatrics. (2019). Diphenhydramine (Benadryl). In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Elk Grove Village, IL: American Academy of Pediatrics; 2019:364-365.
5. Drug Enforcement Administration. (2021). Drugs of Abuse: A DEA Resource Guide. Retrieved from https://www.dea.gov/sites/default/files/2021-01/Drugs%20of%20Abuse%202020%20Edition%20Digital%20Download.pdf
Learn More About Hair Testing Learn More About Nail Testing
- Hair, Nail, and Umbilical Cord Testing for Phenibut, Medetomidine, and Tianeptine
- Umbilical Cord Tissue Testing for SSRIs
- A Comparison of Turnaround-Times for Two Popular Specimen Types Used for Newborn Toxicology: Meconium and Umbilical Cord Tissue
- Using Umbilical Cord Tissue to Identify Prenatal Ethanol Exposure and Co-exposure to Other Commonly Misused Substances
- Toxicology as a Diagnostic Tool to Identify the Misuse of Drugs in the Perinatal Period
- Specimen Delay
- Drug Classes and Neurotransmitters: Amphetamine, Cocaine, and Hallucinogens
- Environmental Exposure Testing for Delta-8 THC, Delta-9 THC, Delta-10 THC, and CBD
- February 2025 (1)
- October 2024 (5)
- March 2024 (1)
- February 2024 (1)
- January 2024 (3)
- December 2023 (1)


