Blog
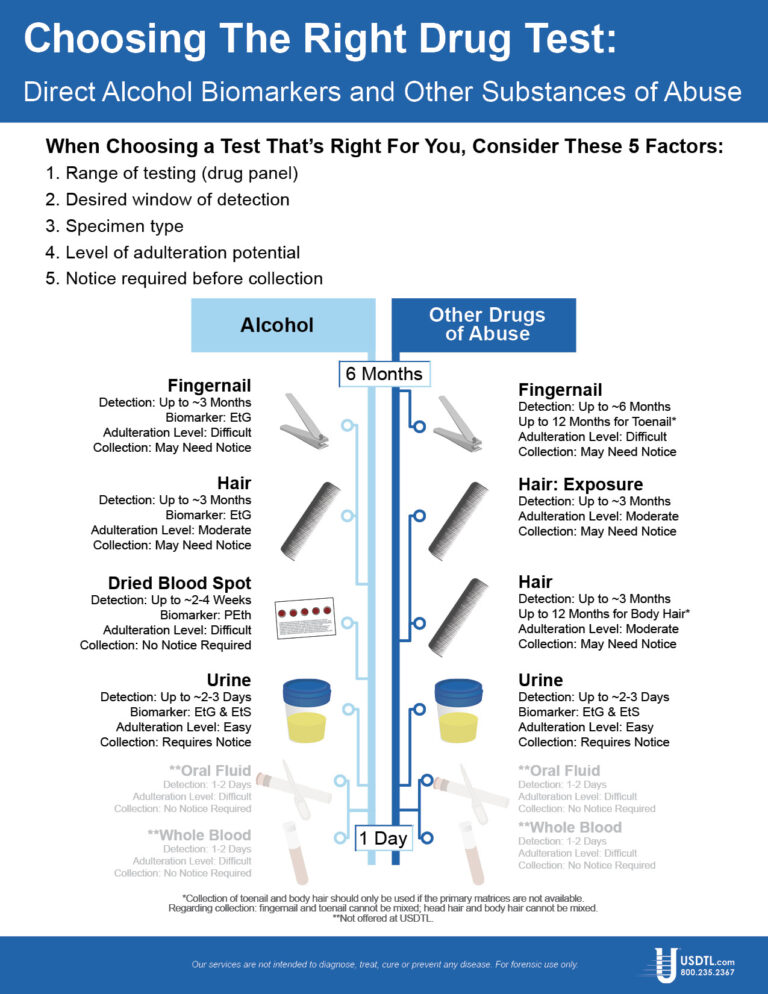
Take a leadership role in drug prevention for our youth. We are proud to participate in Red Ribbon Week, 10/23-10/31. Visit redribbon.org and share their message on social media. Don’t forget to tag them so the message will spread! @redribbonweek #RedRibbonWeek

 Des Plaines, IL – United States Drug Testing Laboratory, Inc. (USDTL), a forensic laboratory specializing in drug and alcohol testing using advanced specimens, has released a new assay to detect zolpidem (Ambien®) use in fingernail and hair specimens. Previously available only in urine and oral-fluid specimens, zolpidem testing in fingernails and hair offers forensic drug testing professionals new, powerful tools to meet their drug testing needs.
Des Plaines, IL – United States Drug Testing Laboratory, Inc. (USDTL), a forensic laboratory specializing in drug and alcohol testing using advanced specimens, has released a new assay to detect zolpidem (Ambien®) use in fingernail and hair specimens. Previously available only in urine and oral-fluid specimens, zolpidem testing in fingernails and hair offers forensic drug testing professionals new, powerful tools to meet their drug testing needs.
Since its approval in 1993 for the treatment of insomnia, zolpidem has become one of the most popular and most prescribed sleep aids. Zolpidem is a sedative-hypnotic medication that affects the same areas of the brain as benzodiazepines. In 2011, 39 million prescriptions for zolpidem products were written in the United States. [1]
Zolpidem use carries some risks of harm, especially when taken in combination with other substances. The number of emergency department visits related to zolpidem grew by 136% from 2004 (12,792) to 2011 (30,149). [2] Other substances were involved in 57% of those ED visits, including benzodiazepines (26%), narcotic pain relievers (25%), and alcohol (14%). [3]
The development of zolpidem testing is driven by USDTL’s ongoing commitment to be a leader in substance abuse and alcohol detection. Zolpidem testing in fingernails and hair will be available on October 1st, 2014.
USDTL has made significant breakthroughs in detecting alcohol and other substances of abuse. They offer a wide range of testing services and specialize in hard to detect substances of abuse, customized assays, and advanced drug testing specimens.
References
1. IMS, Vector One: National (VONA) and Total Patient Tracker (TOT). Year 2011. Extracted June 2011.
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. (May 1, 2013). Emergency Department Visits for Adverse Reactions Involving the Insomnia Medication Zolpidem. Rockville, MD.
3. Substance Abuse and Mental Health Services Administration, Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013.
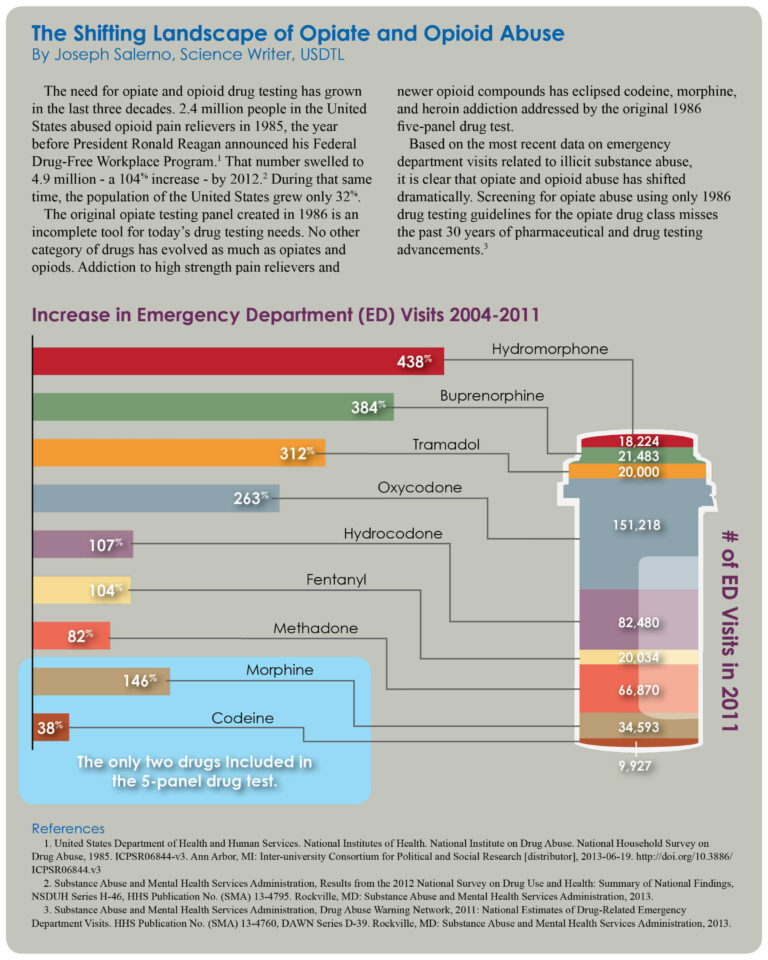
 On August 18, 2014, the pain reliever tramadol (Ultram®) will be scheduled as a Class IV controlled substance by the U.S. Drug Enforcement Administration.1 Tramadol is a synthetic, opioid pain reliever similar in strength to codeine. In 2012, more than 40 million tramadol prescriptions were written in the United States, more than any other opioid medications except for hydrocodone and oxycodone.2
On August 18, 2014, the pain reliever tramadol (Ultram®) will be scheduled as a Class IV controlled substance by the U.S. Drug Enforcement Administration.1 Tramadol is a synthetic, opioid pain reliever similar in strength to codeine. In 2012, more than 40 million tramadol prescriptions were written in the United States, more than any other opioid medications except for hydrocodone and oxycodone.2
In 1994, clinical data suggested tramadol had a very low abuse potential.3 Research has since shown that tramadol can produce a euphoric high similar to oxycodone. At doses greater than prescription levels, tramadol has addiction reinforcing effects similar to morphine and oxycodone.
Between 2004-2012, emergency department visits related to illicit tramadol use increased from 4,800 incidents to more than 16,000.1 Adverse effects of tramadol include sedation, dizziness, respiratory depression, seizures, apnea, and death.4
Withdrawal symptoms often occur following both abrupt and tapered discontinuation of tramadol use, suggesting an issue of drug dependence for tramadol users.
In the year 2000, tramadol was present in only 82 law enforcement drug seizures. This number increased to 1806 by 2012.1 A 2002 study found that 87 out of 140 healthcare professionals testing positive for tramadol use obtained the drug with illegal prescriptions.
Tramadol is available in both single dose (25-100 mg) and extended release (100-300 mg) forms. Tramadol abusers can crush extended release tablets and ingest them for an instant, high-dose euphoria similar to OxyContin but without the cognitive impairment.2
Fingernail testing detects tramadol use for up to six months, while hair provides a three month look-back. Tramadol testing is available in USDTL’s extended drug panels. If you have questions or need more information about tramadol testing, contact our Client Services group at 800•235•2367 or at clientservices@usdtl.com.
References
1. Office of Diversion Control, Drug and Chemical Evaluation Section. (May 2013). Schedules of Controlled Substances: Placement of Tramadol into Schedule IV. Retrieved from https://www.dea.gov/drug-information/drug-scheduling
2. Nadia Awad. (2014). Now What? DEA Tosses Tramadol in Schedule IV. Retrieved from http://www.medpagetoday.com
3. John Fauber. (2013). Killing Pain: Tramadol the “Safe” Drug of Abuse. Retrieved from http://www.medpagetoday.com
4. Senay, E.C., Adams, E.H., et al. (2003). Physical Dependence on Ultram (tramadol hydrochloride): Both opioid-like and atypical withdrawal symptoms occur. Drug and Alcohol Dependence, 69:233-241. DOI: 10.1016/s0376-8716(02)00321-6


- Hair, Nail, and Umbilical Cord Testing for Phenibut, Medetomidine, and Tianeptine
- Umbilical Cord Tissue Testing for SSRIs
- A Comparison of Turnaround-Times for Two Popular Specimen Types Used for Newborn Toxicology: Meconium and Umbilical Cord Tissue
- Using Umbilical Cord Tissue to Identify Prenatal Ethanol Exposure and Co-exposure to Other Commonly Misused Substances
- Toxicology as a Diagnostic Tool to Identify the Misuse of Drugs in the Perinatal Period
- Specimen Delay
- Drug Classes and Neurotransmitters: Amphetamine, Cocaine, and Hallucinogens
- Environmental Exposure Testing for Delta-8 THC, Delta-9 THC, Delta-10 THC, and CBD
- February 2025 (1)
- October 2024 (5)
- March 2024 (1)
- February 2024 (1)
- January 2024 (3)
- December 2023 (1)